Ekukhiqizeni nasekuphileni, ijeli ye-silica ingasetshenziswa ukomisa i-N2, umoya, i-hydrogen, igesi yemvelo [1] njalonjalo.Ngokusho kwe-asidi ne-alkali, i-desiccant ingahlukaniswa ibe: i-acid desiccant, i-alkaline desiccant kanye ne-desiccant engathathi hlangothi [2].Ijeli ye-silica ibonakala iwumshini wokomisa ongathathi hlangothi obonakala womisa i-NH3, i-HCl, i-SO2, njll. Kodwa-ke, ngokombono wesimiso, ijeli ye-silica yakhiwa ukuphelelwa amandla kwamanzi kwe-intermolecular ye-orthosilicic acid, umzimba oyinhloko yi-SiO2, futhi ubuso bucebile ngamaqembu e-hydroxyl (bheka Umfanekiso 1).Isizathu esenza ukuthi ijeli ye-silica ikwazi ukumunca amanzi ukuthi iqembu le-silicon hydroxyl elingaphezulu kwejeli ye-silica lingakha amabhondi e-hydrogen e-intermolecular nama-molecule amanzi, ngakho-ke lingakhanga amanzi futhi ngaleyo ndlela lidlale indima yokomisa.Ijeli ye-silica eshintsha umbala iqukethe ama-cobalt ion, futhi ngemva kokuba amanzi e-adsorption efinyelela ukugcwala, ama-cobalt ion ejeli ye-silica eshintsha umbala abe ama-hydrated cobalt ion, ukuze ijeli ye-silica eluhlaza ibe pink.Ngemuva kokushisisa ijeli ye-silica epinki ku-200 ℃ isikhathi esithile, isibopho se-hydrogen phakathi kwejeli ye-silica nama-molecule wamanzi siyaphuka, futhi ijeli ye-silica eguquliwe izophenduka ibe luhlaza okwesibhakabhaka futhi, ukuze umdwebo wesakhiwo se-silicic acid nejeli ye-silica ikwazi. ziphinde zisetshenziswe njengoba kuboniswe kuMfanekiso 1. Ngakho-ke, njengoba ingaphezulu lejeli ye-silica licebile ngamaqembu e-hydroxyl, ingaphezulu lejeli ye-silica ingase futhi yakhe amabhondi e-hydrogen e-intermolecular ne-NH3 ne-HCl, njll., futhi kungase kungabi khona indlela yokwenza i-desiccant ye-NH3 ne-HCl, futhi awukho umbiko ofanelekile ezincwadini ezikhona.Yaba yini imiphumela?Lesi sihloko senze ucwaningo lokuhlola olulandelayo.
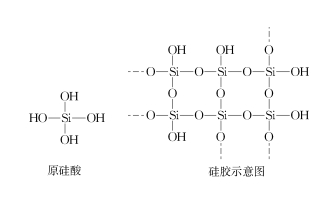
I-FIG.1 Umdwebo wesakhiwo se-ortho-silicic acid nejeli ye-silica
2 Ingxenye Yokuhlola
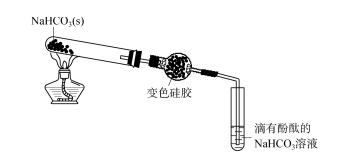
2.1 Ukuhlolwa kobubanzi bokusetshenziswa kwe-silica gel desiccant - I-Ammonia Okokuqala, ijeli ye-silica eshintshiwe yafakwa emanzini a-distilled kanye namanzi e-ammonia agxilile ngokulandelanayo.Ijeli ye-silica eguquliwe iba pink emanzini acwecwe;Ku-ammonia egxilile, i-silicone eshintsha umbala iqala ibe bomvu futhi kancane ishintshe ibe luhlaza okwesibhakabhaka ngokukhanyayo.Lokhu kubonisa ukuthi ijeli ye-silica ingamunca i-NH3 noma i-NH3 ·H2 O ku-ammonia.Njengoba kuboniswe kuMfanekiso 2, i-calcium hydroxide eqinile ne-ammonium chloride kuxutshwe ngokulinganayo futhi kushiselwe eshubhu lokuhlola.Igesi ewumphumela isuswa yi-alkali lime bese kuba ngejeli ye-silica.Umbala wejeli ye-silica eduze nendawo yokungena uba lula (umbala wobubanzi bokufaka isicelo se-silica gel desiccant ku-Figure 2 uyahlolwa - ammonia 73, isigaba sesi-8 sika-2023 ngokuyisisekelo sifana nombala wejeli ye-silica ecwilisiwe. emanzini e-ammonia agxilile), futhi iphepha lokuhlola i-pH alinalo ushintsho olusobala.Lokhu kukhombisa ukuthi i-NH3 ekhiqiziwe ayikafinyeleli ephepheni lokuhlola i-pH, futhi isikhangisiwe ngokuphelele.Ngemva kwesikhathi esithile, misa ukushisa, khipha ingxenye encane yebhola le-silica gel, uyibeke emanzini acwecwe, engeza i-phenolphthalein emanzini, isixazululo siphenduka sibe bomvu, okubonisa ukuthi ijeli ye-silica inomphumela onamandla we-adsorption. I-NH3, ngemva kokuba amanzi ase-distilled evaliwe, i-NH3 ingena emanzini ase-distilled, isisombululo sine-alkaline.Ngakho-ke, ngenxa yokuthi ijeli ye-silica ine-adsorption eqinile ye-NH3, i-ejenti yokomisa i-silicone ayikwazi ukomisa i-NH3.
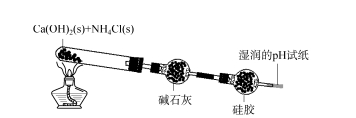
I-FIG.2 Ukuhlolwa kobubanzi bokusetshenziswa kwe-silica gel desiccant - ammonia
2.2 Ukuhlolwa kobubanzi bokusetshenziswa kwe-silica gel desiccant — i-hydrogen chloride iqala ukushisa okuqinile kwe-NaCl ngelangabi lelambu lotshwala ukuze ikhiphe amanzi amanzi ezingxenyeni eziqinile.Ngemuva kokuthi isampula selipholile, i-sulfuric acid egxilile yengezwa ku-NaCl solids ukuze kukhiqizwe inani elikhulu lamabhamuza ngokushesha.Igesi ekhiqiziwe idluliselwa eshubhuni yokomisa eyindilinga equkethe ijeli ye-silica, futhi iphepha lokuhlola i-pH elimanzi lifakwa ekugcineni kweshubhu yokomisa.Ijeli ye-silica ekugcineni ijika ibe luhlaza ngokukhanyayo, futhi iphepha lokuhlola i-pH elimanzi alinalo ushintsho olusobala (bheka uMdwebo 3).Lokhu kubonisa ukuthi igesi ye-HCl ekhiqiziwe ikhangiswa ngokuphelele ngejeli ye-silica futhi ayiphumi emoyeni.
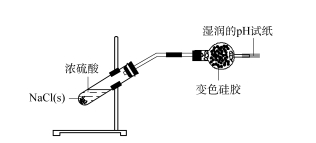
Umfanekiso 3 Ucwaningo mayelana nobubanzi bokusetshenziswa kwe-silica gel desiccant - i-hydrogen chloride
Ijeli ye-silica yakhangisa i-HCl futhi yaphenduka yaba luhlaza ngokukhanyayo yafakwa eshubhuni yokuhlola.Faka ijeli entsha ye-silica eluhlaza okwesibhakabhaka eshubhuni yokuhlola, engeza i-hydrochloric acid egxilile, ijeli ye-silica nayo iba umbala oluhlaza okhanyayo, imibala emibili iyafana ngokuyisisekelo.Lokhu kubonisa igesi yejeli ye-silica kushubhu yokomisa eyindilinga.
2.3 Ukuhlolwa kobubanzi besicelo se-silica gel desiccant - i-sulfur dioxide Ingxube ye-sulfuric acid ehlanganisiwe ne-sodium thiosulfate eqinile (bheka Umfanekiso 4), NA2s2 O3 +H2 SO4 ==Na2 SO4 +SO2 ↑+S↓+H2 O;Igesi ekhiqiziwe idluliswa ngeshubhu yokomisa equkethe ijeli ye-silica eshintshile, ijeli ye-silica eshintshile iba luhlaza okwesibhakabhaka ngokukhanyayo, futhi iphepha le-litmus elihlaza okwesibhakabhaka ekugcineni kwephepha lokuhlola elimanzi alishintshi kakhulu, okubonisa ukuthi igesi ye-SO2 ekhiqiziwe ikhangiswe ngokuphelele i-silica gel ball futhi ayikwazi ukuphunyuka.
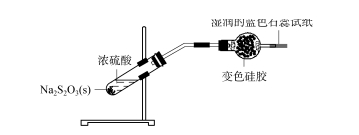
I-FIG.4 Ukuhlolwa kobubanzi bokusetshenziswa kwe-silica gel desiccant - sulphur dioxide
Susa ingxenye ye-silica gel ball bese uyifaka emanzini acwecwe.Ngemuva kokulinganisa okuphelele, thatha inani elincane lokudonsa kwamanzi ephepheni le-litmus eluhlaza okwesibhakabhaka.Iphepha lokuhlola alishintshi kakhulu, libonisa ukuthi amanzi acwengekile awanele ukukhipha i-SO2 kujeli ye-silica.Thatha ingxenye encane yebhola yejeli ye-silica bese uyishisa eshubhu lokuhlola.Faka iphepha elimanzi le-litmus eliluhlaza emlonyeni weshubhu lokuhlola.Iphepha le-litmus eliluhlaza liphenduka libebomvu, okubonisa ukuthi ukufudumeza kwenza igesi ye-SO2 ikhishwe ebholeni lejeli ye-silica, ngaleyo ndlela yenze iphepha le-litmus libe bomvu.Ukuhlola okungenhla kubonisa ukuthi ijeli ye-silica iphinde ibe nomthelela oqinile we-adsorption ku-SO2 noma i-H2 SO3, futhi ayikwazi ukusetshenziselwa ukomisa igesi ye-SO2.
2.4 Ukuhlolwa kobubanzi bokusetshenziswa kwe-silica gel desiccant — I-Carbon dioxide
Njengoba kukhonjisiwe kuMfanekiso 5, isisombululo se-sodium bicarbonate esiconsa i-phenolphthalein sibonakala sibomvu ngokukhanyayo.I-sodium bicarbonate solid iyashiswa futhi ingxube yegesi ewumphumela idluliswa ngeshubhu yokomisa equkethe ama-silica gel spheres omisiwe.Ijeli ye-silica ayishintshi kakhulu futhi i-sodium bicarbonate econsayo ne-phenolphthalein ikhanga i-HCl.I-cobalt ion kujeli ye-silica eshintshile yakha isisombululo esiluhlaza nge-Cl- futhi kancane kancane iba nemibala, okubonisa ukuthi kunenkimbinkimbi yegesi ye-CO2 ekugcineni kweshubhu yokomisa eyindilinga.Ijeli ye-silica eluhlaza ngokukhanyayo ifakwa emanzini acwengekile, futhi ijeli ye-silica eshintshile kancane kancane ishintsha ibe phuzi, okubonisa ukuthi i-HCl ekhangiswe ijeli ye-silica ikhishwe emanzini.Inani elincane lesisombululo esinamanzi esingaphezulu lengezwe esixazululweni se-nitrate esisiliva esifakwe i-asidi ye-nitric ukwenza imvula emhlophe.Inani elincane lesisombululo esinamanzi lilahlwa ephepheni elibanzi lokuhlola i-pH, futhi iphepha lokuhlola liba bomvu, okubonisa ukuthi isixazululo sine-acidic.Ukuhlola okungenhla kubonisa ukuthi ijeli ye-silica ine-adsorption eqinile kugesi we-HCl.I-HCl iyi-molecule ye-polar enamandla, futhi iqembu le-hydroxyl ebusweni bejeli ye-silica libuye libe ne-polarity eqinile, futhi lezi ezimbili zingase zakhe izibopho ze-hydrogen ze-intermolecular noma zibe nokusebenzisana okuqinile kwe-dipole dipole, okuholela kumandla anamandla phakathi kwama-molecular phakathi kobuso be-silica. ijeli ne-HCl molecule, ngakho ijeli ye-silica ine-adsorption eqinile ye-HCl.Ngakho-ke, i-ejenti yokomisa i-silicone ayikwazi ukusetshenziselwa ukomisa ukuphuma kwe-HCl, okungukuthi, ijeli ye-silica ayikhangisi i-CO2 noma i-CO2 kancane kuphela.
I-FIG.5 Ukuhlolwa kobubanzi bokusetshenziswa kwe-silica gel desiccant - carbon dioxide
Ukufakazela ukumenyezelwa kwejeli ye-silica kugesi ye-carbon dioxide, ukuhlolwa okulandelayo kuyaqhubeka.Ibhola lejeli yesilika kushubhu yokomisa eyindilinga lasuswa, futhi ingxenye yahlukaniswa yaba isisombululo se-sodium bicarbonate esiconsa i-phenolphthalein.Isixazululo se-sodium bicarbonate sashintshwa umbala.Lokhu kukhombisa ukuthi ijeli ye-silica ikhangisa i-carbon dioxide, futhi ngemva kokuncibilika emanzini, i-carbon dioxide desorbs ibe isixazululo se-sodium bicarbonate, okwenza i-sodium bicarbonate solution iphele.Ingxenye esele yebhola le-silicone iyashiswa eshubhuni yokuhlola eyomile, futhi igesi ewumphumela idluliselwa kwisisombululo se-sodium bicarbonate econsa nge-phenolphthalein.Ngokushesha, isixazululo se-sodium bicarbonate siyashintsha sisuka kokubomvu ngokukhanyayo sibe singenambala.Lokhu futhi kubonisa ukuthi ijeli ye-silica isenamandla okukhangisa wegesi ye-CO2.Kodwa-ke, amandla okukhangisa wejeli ye-silica ku-CO2 mancane kakhulu kunalawo e-HCl, NH3 kanye ne-SO2, kanye ne-carbon dioxide ingakhangiswa kancane kuphela phakathi nokuhlolwa kuMfanekiso 5. Isizathu sokuthi kungani ijeli ye-silica ingakhangisa kancane i-CO2 kungenzeka ukuthi ijeli ye-silica ne-CO2 zakha amabhondi e-hydrogen e-intermolecular Si — OH… O =C.Ngenxa yokuthi i-athomu ye-carbon emaphakathi ye-CO2 iyi-sp hybrid, kanye ne-athomu ye-silicon kujeli ye-silica iyingxube ye-sp3, i-molecule ye-CO2 ewumugqa ayibambisani kahle nobuso bejeli ye-silica, okuholela ekukhangiseni kwamandla ejeli ye-silica ku-carbon dioxide ngokuqhathaniswa. encane.
3.Ukuqhathanisa phakathi kokuncibilika kwamagesi amane emanzini kanye nesimo se-adsorption ebusweni bejeli ye-silica Kusukela emiphumeleni yokuhlola engenhla, kungabonakala ukuthi ijeli ye-silica inamandla okukhangisa aqinile e-ammonia, i-hydrogen chloride ne-sulphur dioxide, kodwa amandla amancane e-adsorption e-carbon dioxide (bheka Ithebula 1).Lokhu kufana nokuncibilika kwamagesi amane emanzini.Lokhu kungase kube ngenxa yokuthi ama-molecule amanzi aqukethe i-hydroxy-OH, futhi ingaphezulu lejeli ye-silica licebile nge-hydroxyl, ngakho-ke ukuncibilika kwala magesi amane emanzini kufana kakhulu nokukhanyiswa kwawo ebusweni bejeli ye-silica.Phakathi kwamagesi amathathu egesi ye-ammonia, i-hydrogen chloride ne-sulphur dioxide, i-sulphur dioxide inokuncibilika okuncane kakhulu emanzini, kodwa ngemva kokukhangiswa ijeli ye-silica, inzima kakhulu ukucwiliswa phakathi kwamagesi amathathu.Ngemuva kokuthi ijeli ye-silica ikhangisa i-ammonia ne-hydrogen chloride, ingancibilika ngamanzi ancibilikayo.Ngemuva kokuthi igesi ye-sulfur dioxide ikhangiswe ijeli ye-silica, kunzima ukuyikhipha ngamanzi, futhi kufanele ishiswe ukuze ingabikho ebusweni bejeli ye-silica.Ngakho-ke, ukukhangisa kwamagesi amane ebusweni bejeli ye-silica kufanele kubalwe ngokwethiyori.
4 Izibalo zetiyorikhi zokusebenzelana phakathi kwejeli ye-silica namagesi amane kwethulwa kusofthiwe ye-quantumization ORCA [4] ngaphansi kohlaka lwethiyori yokusebenza kwabantu (DFT).Indlela ye-DFT D/B3LYP/Def2 TZVP yasetshenziswa ukubala izindlela zokusebenzisana namandla phakathi kwamagesi ahlukene nejeli ye-silica.Ukuze kube lula ukubala, i-silica gel solids imelwe ama-molecule e-tetrameric orthosilicic acid.Imiphumela yokubala ibonisa ukuthi i-H2 O, NH3 kanye ne-HCl zonke zingakha amabhondi e-hydrogen neqembu le-hydroxyl phezu kwejeli ye-silica (bheka Umfanekiso 6a ~ c).Anamandla okubopha aqinile endaweni yejeli ye-silica (bheka Ithebula 2) futhi akhangeka kalula endaweni yejeli ye-silica.Njengoba amandla okubopha e-NH3 ne-HCl afana nalawo e-H2 O, ukugeza amanzi kungaholela ekuncibilikeni kwala ma-molecule amabili egesi.Ku-molecule ye-SO2, amandla ayo ayisibopho angu-17.47 kJ/mol kuphela, mancane kakhulu kunama-molecule amathathu angenhla.Kodwa-ke, ukuhlolwa kuqinisekisile ukuthi igesi ye-SO2 ikhangiswa kalula kujeli ye-silica, futhi ngisho nokuwasha akukwazi ukuyiqeda, futhi ukufudumeza kuphela okungenza i-SO2 iphume ebusweni bejeli ye-silica.Ngakho-ke, siqagele ukuthi i-SO2 kungenzeka ihlangane ne-H2 O ebusweni bejeli ye-silica ukuze yakhe izingxenyana ze-H2 SO3.Umfanekiso 6e ubonisa ukuthi i-molecule ye-H2 SO3 yakha amabhondi e-hydrogen amathathu nama-athomu e-hydroxyl ne-oksijini ebusweni bejeli ye-silica ngesikhathi esifanayo, futhi amandla okubopha afinyelela ku- -76.63 kJ/mol, okuchaza ukuthi kungani i-SO2 idsorbed ijeli ye-silica kunzima ukuyibalekela ngamanzi.I-non-polar CO2 inamandla okubopha abuthaka kakhulu ngejeli ye-silica, futhi ingakhangiswa kancane ngejeli ye-silica.Nakuba amandla okubopha we-H2 CO3 kanye nejeli ye-silica nawo afinyelele ku--65.65 kJ/mol, izinga lokuguqulwa kwe-CO2 ku-H2 CO3 lalingekho phezulu, ngakho izinga le-adsorption le-CO2 nalo lancishiswa.Kungabonakala kudatha engenhla ukuthi i-polarity ye-molecule yegesi akuyona ukuphela kwendlela yokwahlulela ukuthi ingakhangiswa ngejeli ye-silica, futhi isibopho se-hydrogen esakhiwe nge-silica gel surface yisizathu esiyinhloko sokukhangisa kwayo okuzinzile.
Ukwakhiwa kwejeli ye-silica yi-SiO2 ·nH2 O, indawo enkulu ye-silica gel kanye neqembu elicebile le-hydroxyl ebusweni lenza ijeli ye-silica ingasetshenziswa njengesomisi esingenabuthi esisebenza kahle kakhulu, futhi sisetshenziswa kabanzi ekukhiqizeni nasekuphileni. .Kuleli phepha, kuqinisekiswa ngezici ezimbili zokuhlola nokubala kwethiyori ukuthi ijeli ye-silica ingakhanga i-NH3, i-HCl, i-SO2, i-CO2 namanye amagesi ngamabhondi e-hydrogen e-intermolecular, ngakho ijeli ye-silica ayikwazi ukusetshenziselwa ukomisa lawa magesi.Ukwakhiwa kwejeli ye-silica yi-SiO2 ·nH2 O, indawo enkulu ye-silica gel kanye neqembu elicebile le-hydroxyl ebusweni lenza ijeli ye-silica ingasetshenziswa njengesomisi esingenabuthi esisebenza kahle kakhulu, futhi sisetshenziswa kabanzi ekukhiqizeni nasekuphileni. .Kuleli phepha, kuqinisekiswa ngezici ezimbili zokuhlola nokubala kwethiyori ukuthi ijeli ye-silica ingakhanga i-NH3, i-HCl, i-SO2, i-CO2 namanye amagesi ngamabhondi e-hydrogen e-intermolecular, ngakho ijeli ye-silica ayikwazi ukusetshenziselwa ukomisa lawa magesi.
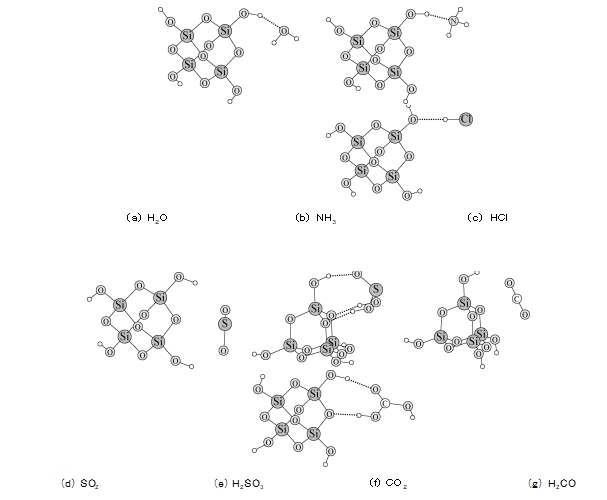
3
I-FIG.6 Izindlela zokusebenzisana phakathi kwama-molecule ahlukene ne-silica gel surface abalwe ngendlela ye-DFT
Isikhathi sokuthumela: Nov-14-2023